Decentralized Institutes of Health
war-on-disease, 1-percent-treaty, medical-research, public-health, peace-dividend, decentralized-trials, dfda, dih, victory-bonds, health-economics, cost-benefit-analysis, clinical-trials, drug-development, regulatory-reform, military-spending, peace-economics, decentralized-governance, wishocracy, blockchain-governance, impact-investing

The NIH spends $47 billion a year. Scientists spend 50-67% of their time writing grant applications instead of doing research. This is not a conspiracy. It’s just what happens when you design a system that rewards asking for money instead of producing results.
Here’s what an alternative might look like.
A decentralized institute of health (DIH) is an alternative institutional design built on four principles.
The Four Principles
Rule 1: Let Patients Decide What Gets Funded
The idea: Give the money directly to patients in the form of subsidies. Let them, in consultation with their doctors, decide which pragmatic clinical trials to join. The trials that attract patients get funded. The ones that don’t, don’t.
This replaces a grant-writing contest with a distributed supercomputer of millions of patients and doctors, all with skin in the game. It’s the collective intelligence of humanity voting with its feet.
Rule 2: Pay for Cures, Not for Promises
The Problem: The NIH pays scientists for writing grant applications. This is like paying a chef for writing a menu instead of cooking a meal.
The alternative: Pay for results. Researchers get paid in two ways:
- Per-patient funding: The more patients who believe in your trial enough to join, the more funding you get.
- Outcome Bounties: Massive, multi-billion dollar prizes for solving the big problems (e.g., “$1 billion to the first team to reverse aging by 10 years”).
In the NIH, you get rich by writing proposals. In a results-based system, you get rich by curing diseases.
Rule 3: Make Corruption Mathematically Impossible
The Problem: Give billions of dollars to humans and they will find creative ways to steal it. This is not cynicism, this is pattern recognition.
The alternative: Replace humans with code. Code is very bad at embezzlement.
- Algorithmic Governance: All funding rules live in smart contracts. You can bribe a committee. You cannot bribe an if-statement.
- No CEO: There’s no one to corrupt because there’s no one. It’s like trying to bribe a vending machine.
- Public Ledger: Every dollar is tracked on a blockchain anyone can read. It’s radical transparency for an industry that has historically preferred opacity.
The goal isn’t to rely on people being virtuous. It’s to design incentives where good outcomes are also the easy outcomes.
Rule 4: Publish Everything (Especially the Failures)
The Problem: Companies and researchers hide their failed experiments. This means scientists around the world waste billions of dollars and decades of time repeating the same mistakes.
The alternative: Make 100% open publication of all data, positive and negative, a condition of funding.
- Every trial, every result, every dataset is published for the world to see.
- AI models continuously scan this global data commons, finding patterns and connections humans would miss.
This doubles research efficiency by preventing the same failure twice. It’s like a group chat where everyone shares what didn’t work, except the group is humanity and the topic is death prevention.
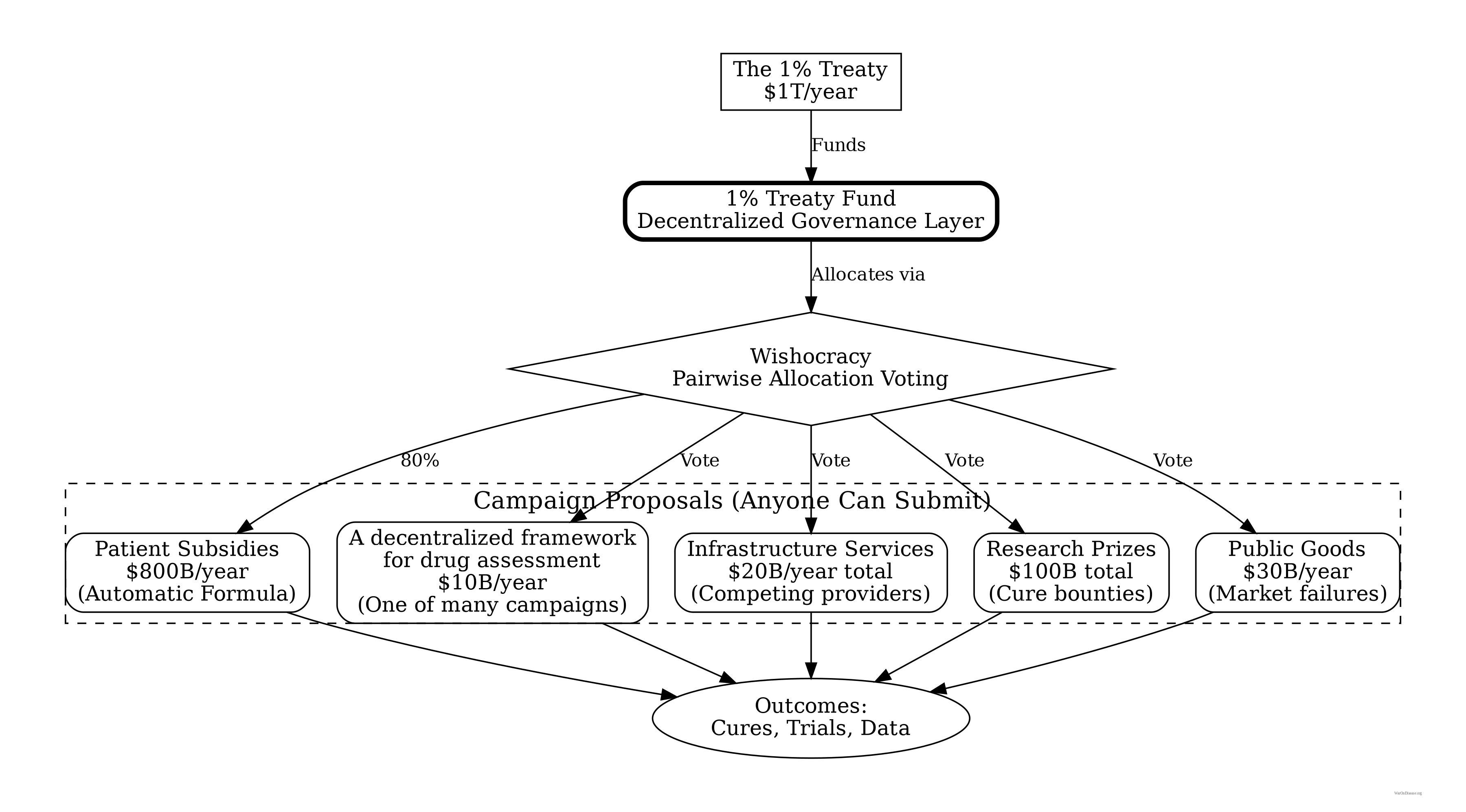
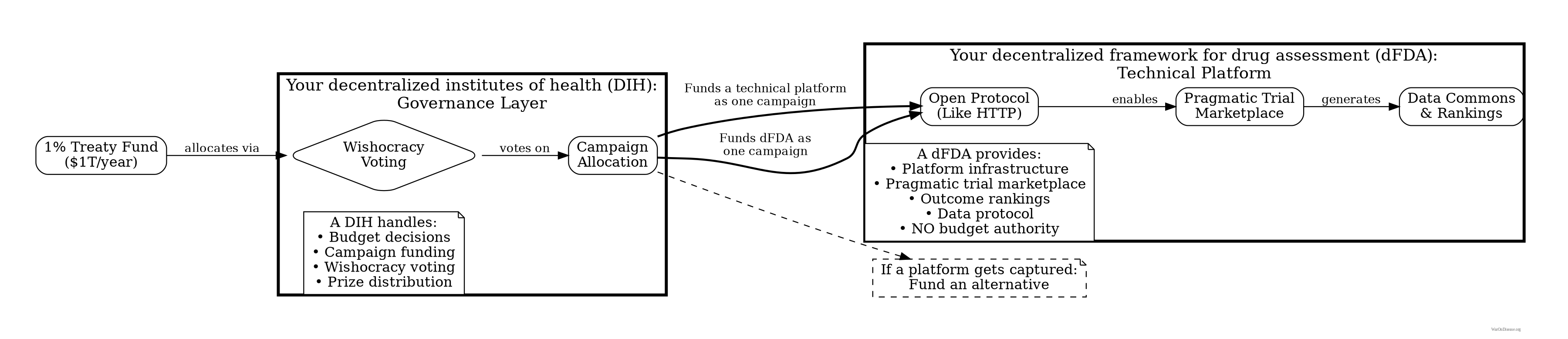
How the Money Flows: The 1% Treaty Fund and Your DIH
The 1% Treaty Fund is the treasury that receives and allocates all funding. Your decentralized institute of health is an implementation pattern for organizations and trials that plug into this fund. Think of the fund as the App Store, and your DIH is one of the apps.
The Architecture
A 1% Treaty Fund
- Holds the treasury (from the 1% Treaty)
- Handles ALL budgeting decisions via Wishocracy
- Funds campaigns, not bureaucracies
- No CEO, no board, no one to corrupt
Your Decentralized Institutes of Health (DIH)
- An implementation pattern for running pragmatic clinical trials.
- Receives funding from the 1% Treaty Fund.
- Competes with other research models for funding.
- The operational layer where the science happens.
Your Decentralized Framework for Drug Assessment (dFDA)
- A pure technical framework/protocol for running pragmatic clinical trials at scale.
- Has no budget authority (it’s a campaign that gets funded by the 1% Treaty Fund).
- Provides marketplace infrastructure for trials.
- One of many campaigns competing for funding.
How It Actually Works
1. Fill the Treasury
A 1% Treaty redirects $27.2B a year from global military budgets into the 1% Treaty Fund. But not all of it reaches Wishocracy:
| Allocation | Share | Amount | Purpose |
|---|---|---|---|
| VICTORY Incentive Alignment Bond investors | 10% |
$2.72B |
Repay the investors who funded the campaign |
| Political incentives (IABs) | 10% |
Keep politicians aligned with curing diseases | |
| 1% Treaty Fund research treasury | $21.7B | Available for allocation via Wishocracy |
2. Anyone Can Propose a Campaign
3. Humanity Allocates via Wishocracy
The global population uses Wishocracy to allocate funds across competing campaigns using pairwise comparisons:
- “What’s more important: decentralized framework for drug assessment development”
- “What’s more important: EHR integration or security audits?”
No committees. No lobbying. Just millions of pairwise preferences aggregated into funding allocations.
4. Campaigns Compete for Funding
- Patient subsidies: Automatically funded (formulaic, ~80% of treasury)
- Infrastructure campaigns: Multiple providers compete (a decentralized framework for drug assessment (dFDA), data storage, integrations)
- Research prizes: Bounties for specific breakthroughs
- Public goods: Market failures that need direct funding
5. Results Determine Future Funding
Campaigns that deliver impact get continued funding. Failures get defunded. It’s venture capital, but for not dying.
Why This Prevents Capture
Traditional centralized system: Concentrated decision-making → lobbying targets
Decentralized alternative
- No director to bribe
- Millions vote via pairwise comparisons
- Open-source allocation algorithms
- Anyone can fork if captured
- Cost to corrupt: Prohibitively high
Risk: “What if your decentralized framework for drug assessment becomes the new FDA?”
Mitigation: Such a framework has no budget authority. It’s just a campaign competing for funding from the 1% Treaty Fund. If it gets captured, fund a competing framework instead.
Projected Efficiency Gains
If the mechanism design works as intended, a decentralized institute of health could achieve significant efficiency improvements over the current system. The following projections are theoretical and depend on successful implementation.
Projected Performance Metrics (Theoretical)
| Metric | NIH (Current) | A DIH (Theoretical) | Potential Improvement |
|---|---|---|---|
| Payment trigger | Grant approved | Results achieved | Incentive alignment |
| Cost per QALY | $50,000-$150,000 | $5,000-$15,000 | ~10x reduction (theoretical) |
| Overhead percentage | 50-67% | <10% | ~6x reduction in overhead |
| Time to treatment | 10-17 years | 2-4 years | ~4x faster (theoretical) |
| Publication rate | ~50% (positive results only) | 100% (all results) | 2x knowledge base |
| Trials run annually | ~5,000 major trials | 50,000-500,000 trials | 10-100x scale (theoretical) |
| Researcher time on research | 33-50% | >90% | ~2x productivity |
How the Efficiency Gains Could Compound
If these improvements are achieved independently, they could multiply:
- ~10x cost reduction per QALY through elimination of overhead
- ~2x knowledge multiplier from mandatory publication of all results
- ~4x speed multiplier from cutting bureaucratic delays
- ~10-100x scale from distributed trial infrastructure (a decentralized framework for drug assessment)
Theoretical ceiling (if everything works): 10 × 2 × 4 × 10 = 800x improvement in QALY production rate
This is obviously a theoretical maximum. Real-world implementation would face friction, resistance, and unforeseen problems. But even achieving 10% of this improvement would represent a significant advance.
What This Could Mean in Practice
A well-functioning decentralized institute of health (DIH), combined with your decentralized framework for drug assessment (dFDA) and operating on a 1% Treaty budget of $27.2B annually, could theoretically produce more QALYs than the entire current global pragmatic clinical trials output.
If the theoretical efficiency gains are even partially realized, this could eventually lead to:
- Significantly improved cancer survival rates
- Aging research accelerated (whether “reversible” is another question)
- Rare diseases becoming economically viable to research
- Personalized medicine becoming more accessible
The core hypothesis: Systems that pay for results tend to produce more results than systems that pay for proposals. Whether this hypothesis holds for medical research at scale is what we’re proposing to test.
That’s the theory. The rest of this guide explains how you’d actually build it.